Science
Related: About this forumSome Interesting Stuff on Deep Eutectics.
We are having a remarkable - and appreciated - cold spell here in the Northeast, may it kill some pine beetle larvae and mosquito eggs.
While it will require putting up some insipid remarks from very stupid and ignorant people - like for instance our very stupid and increasing senile orange so called "President - about the non-reality of "global warming," weather like this, as wonderful as it may be for the ecosystem overall, does inspire some consideration of an environmental consequence which is seldom considered, specifically, the issue of salt flows.
The Fahrenheit scale still in use in a prominent North American and increasingly backward nation is based on two points 0 F and 100 F that were defined incorrectly, the first being the eutectic point of the best known salt, sodium chloride, NaCl, and the latter on the supposed normal homothermic temperature of a human being. On the scale currently used as the "fahrenheit scaled" both are actually wrong. The "normal" body temperature of a human being, with some minor variations is 98.6 F The eutectic point of a sodium chloride/water solution is actually -21.1C which translates to -5.98 F, with a weight % of 23.3% NaCl, as shown in the phase diagram below from a Purdue University Website:
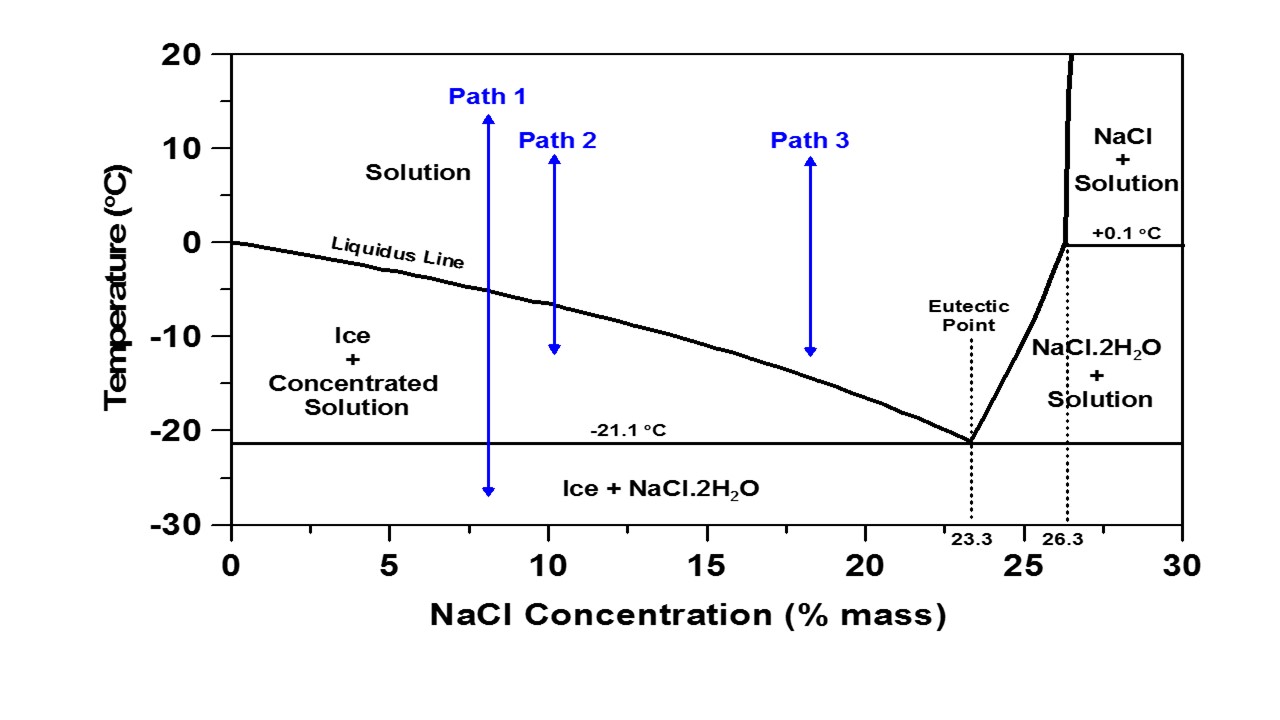
Whatever. I confess that while I do all my thinking about science on the more widely used centigrade scale, and calculations on the thermodynamic Kelvin scale, I still - being one of those awful "baby boomer" people - still think about the weather in fahrenheit, although I wish I didn't.
One of the things that really bugs me - although I hypocritically use it, much as I use the two dangerous fossil fuels I so despise, natural gas and gasoline - is the habit of pouring salt on various forms of pavement. The reason is that I have to wonder where it goes, since salt flows are actually a critical issue environmentally.
This cold weather has caused me to reflect on this issue as did a paper I encountered in the primary scientific literature, this one:
Unconventional Deep Eutectic Solvents: Aqueous Salt Hydrates
This paper is about the use of "deep eutectics" as heat transfer media in closed systems, so it is not necessarily about alternatives in pavement ice melts.
If one accesses the paper, one will find in table 3 a list of eutectic inorganic salts and their eutectic temperatures. Notable is the second most utilized salt for melting ice on pavement, calcium chloride, which has a eutectic point considerably lower than that of the sodium chloride version, specifically -49.8C, which is -57.6 F. I don't think that this is a particularly benign salt either in an environmental sense, but those that are better are often more noxious.
Examples include KOH, sodium hydroxide (the solid form will actually generate heat when exposed to ice or water) which has a eutectic temperature of -65.2C or -84.5 F, and Calcium perchlorate, which has a eutectic point of -74.6C or -102 F.
KOH of course will dissolve flesh (and any other living thing, as well as many non-living things.) Exposed to the atmosphere it rapidly takes up carbon dioxide to form carbonates.
Perchlorates are environmental pollutants associated with the exhaust of some rockets and fireworks; they are oxidants and also interfere with iodine metabolism.
Probably, from my perspective, the least obnoxious alternative would be magnesium acetate, since acetate is readily metabolized and magnesium is a constituent of some fertilizers.
Where metabolism is concerned, probably an interesting set of eutectics are represented by choline/urea systems - choline being a constituent of most living cells, as is urea.
A paper on the physical chemistry of the choline/urea/water system has just been accepted for publication and now appears online:
First Principles Molecular Dynamics Study of a Deep Eutectic Solvent: Choline Chloride/Urea and Its Mixture with Water (J. Phys. Chem B. Just accepted, accessed 12/31/17)
Another, from the wonderful accurate and precise journal Chem. Eng. Data appears here: Molar Enthalpy of Mixing for Choline Chloride/Urea Deep Eutectic Solvent + Water System
While being biological molecules these are less obnoxious on land that inorganic salts, it's pretty clear to me that they would create huge problems in the already intractable and serious problem with the nitrogen cycle, which is responsible for the destruction of huge bodies of water owing to eutrophication, and are involved in the atmospheric accumulation of the climate forcing and ozone destroying gas nitrous oxide, N2O, "laughing gas."
(It's no laughing matter.)
It's time to go to New Year's Parties.
Have the happiest and prosperous New Year, irrespective of the Republican's efforts to limit happiness and prosperity to the most venal, selfish, and useless people on the planet.
elfin
(6,262 posts)Living in the northern part of the US, we have become so used to periodic, long, deep cold that kills nasty insects that we are astounded when it isn't deep enough or long enough to do the job.
When it lessens, we are amazed at "blooms" of critters that have managed to survive and thrive into the summer. The summer of '17 was a "bloom" in many uncomfortable ways.
I think (hope) this year, despite its discomfort now will bring a more comfortable summer, with fewer infestations.
eppur_se_muova
(36,305 posts)... to melt the ice on sidewalks. The runoff "burned" the grass, leaving big dead, brown patches. ![]()